Chemotherapy plus camrelizumab versus chemotherapy alone as neoadjuvant treatment for resectable esophageal squamous cell carcinoma (ESCORT-NEO): A multi-center, randomized phase III trial.
現地レポート
Live Short Cuts
聴講直後のホットな状態のまま現地サンフランシスコでコメント収録。ライブなインプレッションを提供します。
A randomized controlled phase III trial comparing thoracoscopic esophagectomy and open esophagectomy for thoracic esophageal cancer: JCOG1409 (MONET trial).
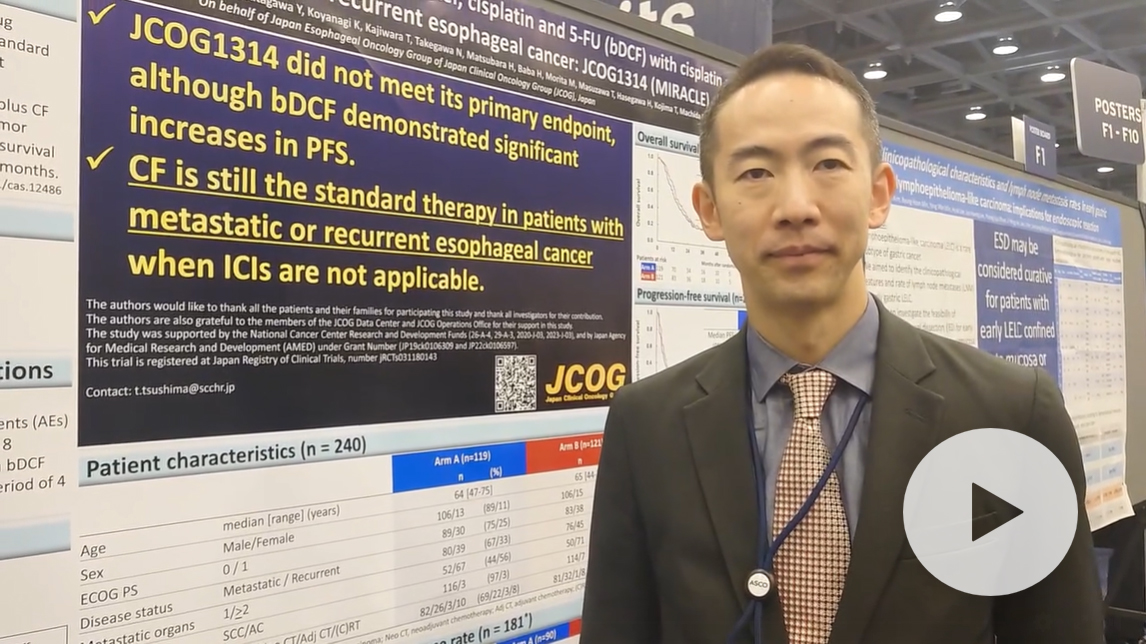
A randomized phase III study comparing 2-weekly docetaxel, cisplatin and 5-FU (bDCF) with cisplatin and 5-FU (CF) in patients with metastatic or recurrent esophageal cancer: JCOG1314 (MIRACLE).
SKYSCRAPER-08: A phase III, randomized, double-blind, placebo-controlled study of first-line (1L) tiragolumab (tira) + atezolizumab (atezo) and chemotherapy (CT) in patients (pts) with esophageal squamous cell carcinoma (ESCC).
(言及した順番)
Abstract #249
A randomized controlled phase III trial comparing thoracoscopic esophagectomy and open esophagectomy for thoracic esophageal cancer: JCOG1409 (MONET trial).
Abstract #LBA248
A two-arm randomized open-label prospective design superiority phase III clinical trial to compare the efficacy of docetaxel-oxaliplatin-capecitabine/5 fluorouracil (DOC/F) followed by docetaxel versus CAPOX/mFOLFOX-7 in advanced gastric cancers (DOC-GC study).
Abstract #322
A randomized phase III study comparing 2-weekly docetaxel, cisplatin and 5-FU (bDCF) with cisplatin and 5-FU (CF) in patients with metastatic or recurrent esophageal cancer: JCOG1314 (MIRACLE).
Abstract #LBA244
Chemotherapy plus camrelizumab versus chemotherapy alone as neoadjuvant treatment for resectable esophageal squamous cell carcinoma (ESCORT-NEO): A multi-center, randomized phase III trial.
Abstract #245
SKYSCRAPER-08: A phase III, randomized, double-blind, placebo-controlled study of first-line (1L) tiragolumab (tira) + atezolizumab (atezo) and chemotherapy (CT) in patients (pts) with esophageal squamous cell carcinoma (ESCC).
Abstract #251
A quality-adjusted time without symptoms and toxicity (Q-TWiST) analysis comparing nivolumab plus ipilimumab (N+I) or nivolumab plus chemotherapy (N+CT) versus CT in patients (pts) with advanced esophageal squamous cell carcinoma (ESCC): CheckMate 648.
Abstract #252
Nivolumab (NIVO) plus (+) chemotherapy (chemo) or ipilimumab (IPI) vs chemo as 1L treatment for advanced esophageal squamous cell carcinoma (ESCC): First comprehensive biomarker analyses from CheckMate 648.
Abstract #247
Pembrolizumab plus FLOT vs FLOT as neoadjuvant and adjuvant therapy in locally advanced gastric and gastroesophageal junction cancer: Interim analysis of the phase 3 KEYNOTE-585 study.
Abstract #LBA246
Pathological complete response (pCR) to 5-fluorouracil, leucovorin, oxaliplatin and docetaxel (FLOT) with or without durvalumab (D) in resectable gastric and gastroesophageal junction cancer (GC/GEJC): Subgroup analysis by region from the phase 3, randomized, double-blind MATTERHORN study.





更新履歴
2024-01-22
動画を公開しました
2024-01-20
動画を公開しました